First synthesis of a antimony nitride crystal
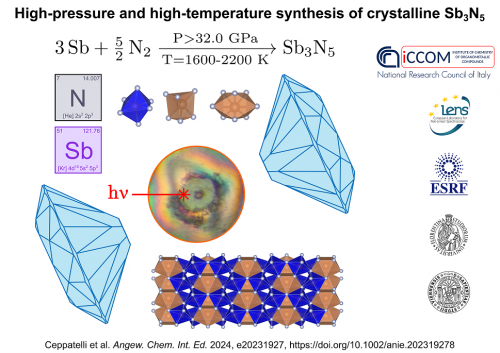 An international research group, coordinated by the Institute of Chemistry of Organometallic Compounds of the National Research Council (CNR-ICCOM) of Sesto Fiorentino (Florence) and by the European Laboratory of Non-Linear Spectroscopies (LENS) of the University of Florence, managed to synthesize for the first time a compound whose existence has long been hypothesized, but never experimentally observed: crystalline antimony nitride, with the chemical formula Sb3N5. The new compound was discovered by activating a direct chemical reaction between antimony and nitrogen under high pressure and high temperature conditions. This synthesis method makes it possible to reduce interatomic distances, thanks to the effect of high pressure, and to overcome the energy barriers for activating the reaction thanks to the effect of high temperature, favoring reaction paths that are otherwise inaccessible to ambient pressure. Using diamond anvil cells to generate high-pressure conditions (32 GPa, approximately 320,000 times the atmospheric pressure) and infrared laser radiation to heat the sample to the required high temperature (1600-2200 K), the team of research managed to activate a direct chemical reaction between elemental antimony and molecular nitrogen which made it possible to synthesize for the first time a crystalline antimony nitride (Sb3N5), whose structure was determined by single crystal X-ray diffraction at the ESRF synchrotron (Grenoble, France).
An international research group, coordinated by the Institute of Chemistry of Organometallic Compounds of the National Research Council (CNR-ICCOM) of Sesto Fiorentino (Florence) and by the European Laboratory of Non-Linear Spectroscopies (LENS) of the University of Florence, managed to synthesize for the first time a compound whose existence has long been hypothesized, but never experimentally observed: crystalline antimony nitride, with the chemical formula Sb3N5. The new compound was discovered by activating a direct chemical reaction between antimony and nitrogen under high pressure and high temperature conditions. This synthesis method makes it possible to reduce interatomic distances, thanks to the effect of high pressure, and to overcome the energy barriers for activating the reaction thanks to the effect of high temperature, favoring reaction paths that are otherwise inaccessible to ambient pressure. Using diamond anvil cells to generate high-pressure conditions (32 GPa, approximately 320,000 times the atmospheric pressure) and infrared laser radiation to heat the sample to the required high temperature (1600-2200 K), the team of research managed to activate a direct chemical reaction between elemental antimony and molecular nitrogen which made it possible to synthesize for the first time a crystalline antimony nitride (Sb3N5), whose structure was determined by single crystal X-ray diffraction at the ESRF synchrotron (Grenoble, France).
For more information (in Italian):
